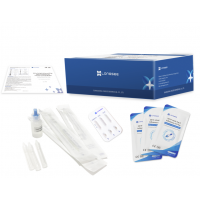
Product Components
| Component | 25 T/kit | 50 T/kit | 100 T/kit | 200 T/kit | |
|---|---|---|---|---|---|
| 1 | Test Cartridge | 25 pcs | 50 pcs | 100 pcs | 200 pcs |
| 2 | One-off Swab | 25 pcs | 50 pcs | 100 pcs | 200 pcs |
| 3 | Extraction Tube and Dripper | 25 pcs | 50 pcs | 100 pcs | 200 pcs |
| 4 | Sample Extraction Buffer | 15 mL | 30 mL | 60 mL | 120 mL |
Storage Condition & Expiration Date
1. 12 months at 4-35 °C
2. Do not freeze. The test card should be used as soon as possible within 1 hour after the aluminium foil bag is opened.
Instructions for Use
Note: Reading the IFU carefully before using this reagent, and perform testing strictly in accordance with the use manual, otherwise reliable results cannot be guaranteed.
1. Before testing
Equilibrate the test cartridge and sample to room temperature.It is recommended to unpack the test card after equilibrating to room temperature and use it as soon as possible within the validity period to prevent the test card from humidity.
2. Extract
1)Transfer 400 μL (about 10 drops) sample extraction buffer to the extraction tube vertically.
2)Insert the swab which has collected secretions into the sample extraction buffer,rotate about 10 times and stay still at room temperature for 5 minutes.
3) Squeeze the swab tip to keep the liquid in the tube as much as possible and take out the one-off swab.
4) Cover the dripper
3. Loading
Tear off the aluminum foil packaging bag, take out the test card, lay it flat on a horizontal table, drop 3~4 drops (about 100 μL) of the sample vertically into the sample hole, and start timing.
4. Detect
Observe the display result in 15 minutes after adding the sample, and the test result is invalid after 20 minutes.

Interpretation of the Result
1. Invalid: When there is no red line in the quality control area (C), the test is invalid. It is recommended to retest with a new test card, paying particular attention to whether the sample volume is sufficient.
2. Positive: A red reaction line appears in the detection area (T) and the qualitycontrol area (C) of the lane COVID-19,Flu A and Flu B respectively
3. Negative: A red line only appears in the quality control area (C).